- Thyroid
- A Multicenter, Randomized, Controlled Trial for Assessing the Usefulness of Suppressing Thyroid Stimulating Hormone Target Levels after Thyroid Lobectomy in Low to Intermediate Risk Thyroid Cancer Patients (MASTER): A Study Protocol
-
Eun Kyung Lee, Yea Eun Kang, Young Joo Park, Bon Seok Koo, Ki-Wook Chung, Eu Jeong Ku, Ho-Ryun Won, Won Sang Yoo, Eonju Jeon, Se Hyun Paek, Yong Sang Lee, Dong Mee Lim, Yong Joon Suh, Ha Kyoung Park, Hyo-Jeong Kim, Bo Hyun Kim, Mijin Kim, Sun Wook Kim, Ka Hee Yi, Sue K. Park, Eun-Jae Jung, June Young Choi, Ja Seong Bae, Joon Hwa Hong, Kee-Hyun Nam, Young Ki Lee, Hyeong Won Yu, Sujeong Go, Young Mi Kang, MASTER study group
-
Endocrinol Metab. 2021;36(3):574-581. Published online May 26, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.943
-
-
6,300
View
-
268
Download
-
8
Web of Science
-
11
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Postoperative thyroid stimulating hormone (TSH) suppression therapy is recommended for patients with intermediate- and high-risk differentiated thyroid cancer to prevent the recurrence of thyroid cancer. With the recent increase in small thyroid cancer cases, the extent of resection during surgery has generally decreased. Therefore, questions have been raised about the efficacy and long-term side effects of TSH suppression therapy in patients who have undergone a lobectomy.
Methods
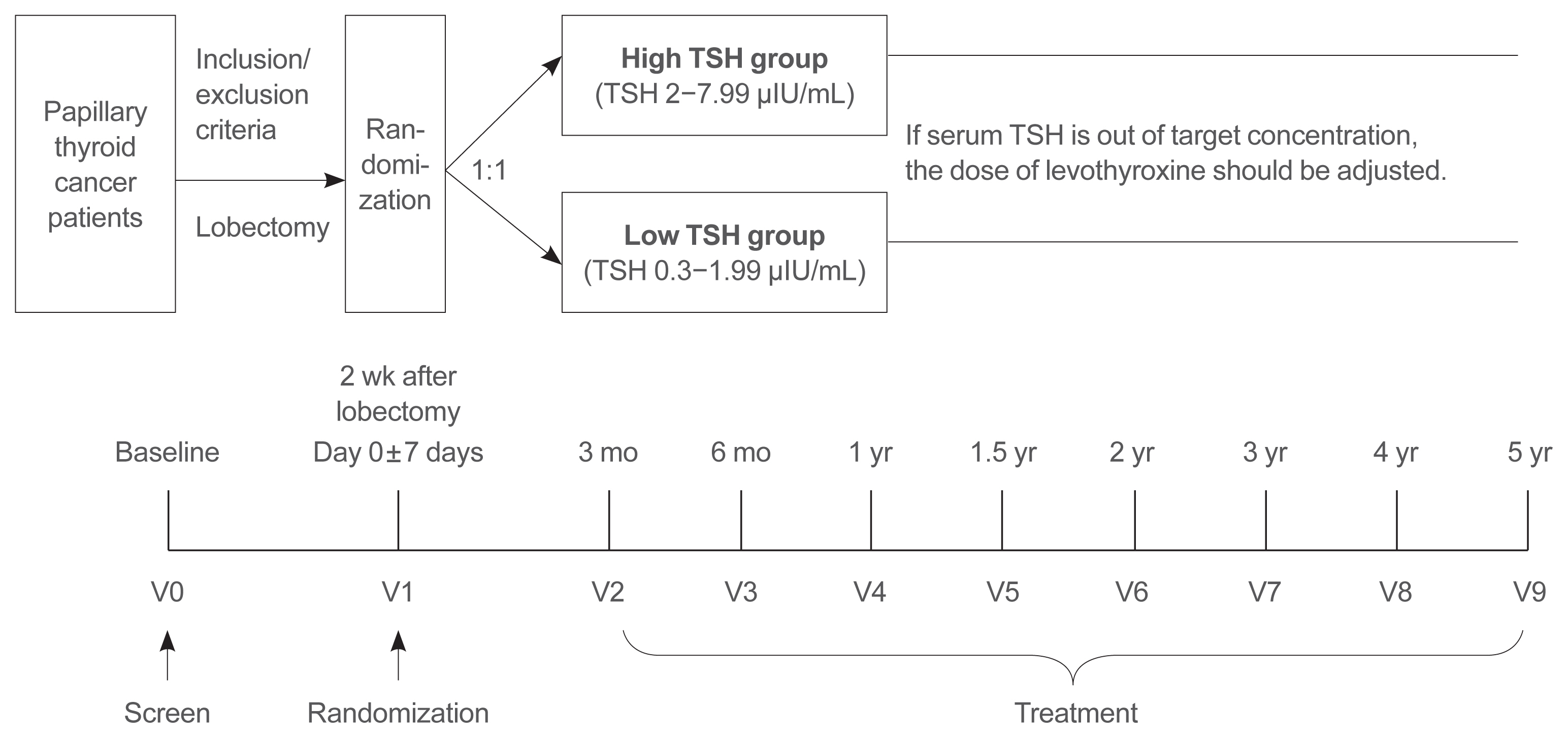
This is a multicenter, prospective, randomized, controlled clinical trial in which 2,986 patients with papillary thyroid cancer are randomized into a high-TSH group (intervention) and a low-TSH group (control) after having undergone a lobectomy. The principle of treatment includes a TSH-lowering regimen aimed at TSH levels between 0.3 and 1.99 μIU/mL in the low-TSH group. The high-TSH group targets TSH levels between 2.0 and 7.99 μIU/mL. The dose of levothyroxine will be adjusted at each visit to maintain the target TSH level. The primary outcome is recurrence-free survival, as assessed by neck ultrasound every 6 to 12 months. Secondary endpoints include disease-free survival, overall survival, success rate in reaching the TSH target range, the proportion of patients with major cardiovascular diseases or bone metabolic disease, the quality of life, and medical costs. The follow-up period is 5 years.
Conclusion
The results of this trial will contribute to establishing the optimal indication for TSH suppression therapy in low-risk papillary thyroid cancer patients by evaluating the benefit and harm of lowering TSH levels in terms of recurrence, metabolic complications, costs, and quality of life.
-
Citations
Citations to this article as recorded by  - Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)
Ja Kyung Lee, Eu Jeong Ku, Su-jin Kim, Woochul Kim, Jae Won Cho, Kyong Yeun Jung, Hyeong Won Yu, Yea Eun Kang, Mijin Kim, Hee Kyung Kim, Junsun Ryu, June Young Choi
Annals of Surgical Treatment and Research.2024; 106(1): 19. CrossRef - Clinical impact of coexistent chronic lymphocytic thyroiditis on central lymph node metastasis in low- to intermediate-risk papillary thyroid carcinoma: The MASTER study
Da Beom Heo, Ho-Ryun Won, Kyung Tae, Yea Eun Kang, Eonju Jeon, Yong Bae Ji, Jae Won Chang, June Young Choi, Hyeong Won Yu, Eu Jeong Ku, Eun Kyung Lee, Mijin Kim, Jun-Ho Choe, Bon Seok Koo
Surgery.2024; 175(4): 1049. CrossRef - Dynamic Changes in Treatment Response af-ter 131I in Differentiated Thyroid Cancer and Their Relationship with Recurrence Risk Stratification and TNM Staging
璐 狄
Advances in Clinical Medicine.2024; 14(03): 1083. CrossRef - ASO Author Reflections: Active Surveillance may be Possible in Patients with T1b Papillary Thyroid Carcinoma Over 55 Years of Age Without High-Risk Features on Preoperative Examinations
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2254. CrossRef - Outcomes and Trends of Treatments in High‐Risk Differentiated Thyroid Cancer
Arash Abiri, Khodayar Goshtasbi, Sina J. Torabi, Edward C. Kuan, William B. Armstrong, Tjoson Tjoa, Yarah M. Haidar
Otolaryngology–Head and Neck Surgery.2023; 168(4): 745. CrossRef - Current Controversies in Low-Risk Differentiated Thyroid Cancer: Reducing Overtreatment in an Era of Overdiagnosis
Timothy M Ullmann, Maria Papaleontiou, Julie Ann Sosa
The Journal of Clinical Endocrinology & Metabolism.2023; 108(2): 271. CrossRef - Age-Dependent Clinicopathological Characteristics of Patients with T1b Papillary Thyroid Carcinoma: Implications for the Possibility of Active Surveillance
Ho-Ryun Won, Eonju Jeon, Da Beom Heo, Jae Won Chang, Minho Shong, Je Ryong Kim, Hyemi Ko, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Kyong Hye Joung, Ji Min Kim, Younju Lee, Sung-Woo Kim, Young Ju Jeong, Yong Bae Ji, Kyung Tae, Bon Seok Koo
Annals of Surgical Oncology.2023; 30(4): 2246. CrossRef - Potential impact of obesity on the aggressiveness of low- to intermediate-risk papillary thyroid carcinoma: results from a MASTER cohort study
Mijin Kim, Yae Eun Kang, Young Joo Park, Bon Seok Koo, Eu Jeong Ku, June Young Choi, Eun Kyung Lee, Bo Hyun Kim
Endocrine.2023; 82(1): 134. CrossRef - Differentiated thyroid cancer: a focus on post-operative thyroid hormone replacement and thyrotropin suppression therapy
Benjamin J. Gigliotti, Sina Jasim
Endocrine.2023; 83(2): 251. CrossRef - Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma
Mi Rye Bae, Sung Hoon Nam, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim
Endocrine.2022; 75(2): 487. CrossRef - The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research
Kyungsik Kim, Mijin Kim, Woojin Lim, Bo Hyun Kim, Sue K. Park
Endocrinology and Metabolism.2021; 36(4): 725. CrossRef
- Clinical Study
- Lactate Dehydrogenase A as a Potential New Biomarker for Thyroid Cancer
-
Eun Jeong Ban, Daham Kim, Jin Kyong Kim, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Kunhong Kim
-
Endocrinol Metab. 2021;36(1):96-105. Published online February 24, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.819
-
-
5,830
View
-
188
Download
-
14
Web of Science
-
13
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Several cancers show increased levels of lactate dehydrogenase A (LDHA), which are associated with cancer progression. However, it remains unclear whether LDHA levels are associated with papillary thyroid cancer (PTC) aggressiveness or with the presence of the PTC prognostic marker, the BRAFV600E mutation. This study aimed to evaluate the potential of LDHA as a PTC prognostic marker.
Methods
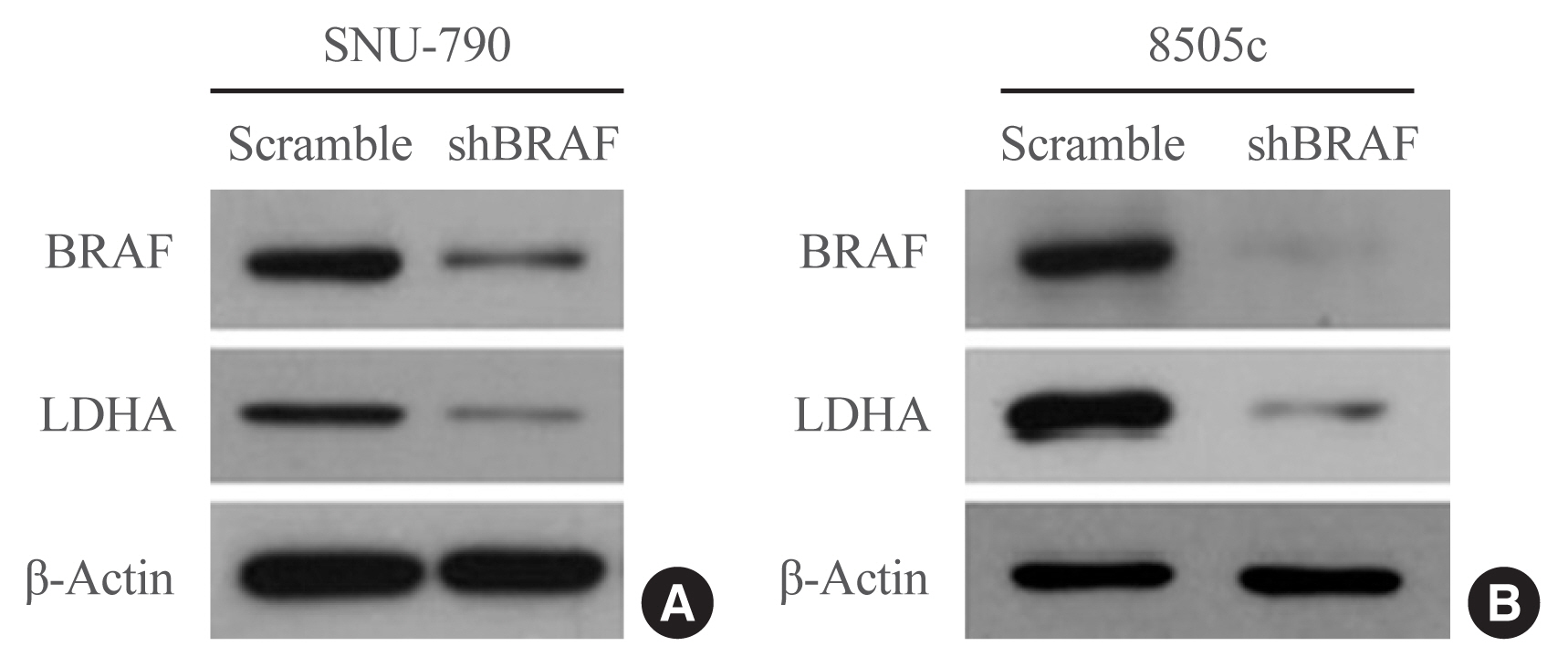
LDHA expression was examined in 83 PTC tissue specimens by immunohistochemistry. Human thyroid cell lines were genetically manipulated to overexpress BRAFV600E or were treated with a BRAF-specific short hairpin RNA (shBRAF), whose effects on LDHA expression were evaluated by Western blotting. Data from 465 PTC patients were obtained from The Cancer Genome Atlas (TCGA) database and analyzed to validate the in vitro results.
Results
LDHA was aberrantly overexpressed in PTC. Intense immunostaining for LDHA was observed in PTC specimens carrying mutated BRAF, whereas the intensity was less in wild-type BRAF samples. Overexpression of BRAFV600E resulted in LDHA upregulation, whereas treatment with shBRAF downregulated LDHA in human thyroid cell lines. Furthermore, LDHA mRNA expression was significantly elevated and associated with BRAFV600E expression in thyroid cancer tissues from TCGA database. Additionally, LDHA overexpression was found to be correlated with aggressive clinical features of PTC, such as lymph node metastases and advanced tumor stages.
Conclusion
LDHA overexpression is associated with the BRAFV600E mutation and an aggressive PTC behavior. Therefore, LDHA may serve as a biomarker and therapeutic target in PTC.
-
Citations
Citations to this article as recorded by  - Integrated proteogenomic and metabolomic characterization of papillary thyroid cancer with different recurrence risks
Ning Qu, Di Chen, Ben Ma, Lijun Zhang, Qiuping Wang, Yuting Wang, Hongping Wang, Zhaoxian Ni, Wen Wang, Tian Liao, Jun Xiang, Yulong Wang, Shi Jin, Dixin Xue, Weili Wu, Yu Wang, Qinghai Ji, Hui He, Hai-long Piao, Rongliang Shi
Nature Communications.2024;[Epub] CrossRef - Peripheral lymphocytes and lactate dehydrogenase correlate with response and survival in head and neck cancers treated with immune checkpoint inhibitors
Cassie Pan, Qian Vicky Wu, Jenna Voutsinas, Jeffrey J. Houlton, Brittany Barber, Zain H. Rizvi, Emily Marchiano, Neal Futran, George E. Laramore, Jay J. Liao, Upendra Parvathaneni, Renato G. Martins, Jonathan R. Fromm, Cristina P. Rodriguez
Cancer Medicine.2023; 12(8): 9384. CrossRef - LncRNA GLTC targets LDHA for succinylation and enzymatic activity to promote progression and radioiodine resistance in papillary thyroid cancer
Liang Shi, Rui Duan, Zhenhua Sun, Qiong Jia, Wenyu Wu, Feng Wang, Jianjun Liu, Hao Zhang, Xue Xue
Cell Death & Differentiation.2023; 30(6): 1517. CrossRef - Integrated analysis of circulating and tissue proteomes reveals that fibronectin 1 is a potential biomarker in papillary thyroid cancer
Guochao Ye, Xiaomei Zhang, Mansheng Li, Zixiang Lin, Yongcan Xu, Haoru Dong, Jie Zhou, Jiaqi Zhang, Sheng Wang, Yunping Zhu, Xiaobo Yu, Xu Qian
BMC Cancer.2023;[Epub] CrossRef - Targeting metabolism by B-raf inhibitors and diclofenac restrains the viability of BRAF-mutated thyroid carcinomas with Hif-1α-mediated glycolytic phenotype
Marianna Aprile, Simona Cataldi, Caterina Perfetto, Antonio Federico, Alfredo Ciccodicola, Valerio Costa
British Journal of Cancer.2023; 129(2): 249. CrossRef - circNFATC3 facilitated the progression of oral squamous cell carcinoma via the miR-520h/LDHA axis
Hongguo Xie, Xiaopeng Lu
Open Medicine.2023;[Epub] CrossRef - The potential role of reprogrammed glucose metabolism: an emerging actionable codependent target in thyroid cancer
Sai-li Duan, Min Wu, Zhe-Jia Zhang, Shi Chang
Journal of Translational Medicine.2023;[Epub] CrossRef - CENPE and LDHA were potential prognostic biomarkers of chromophobe renal cell carcinoma
Hui-feng Wu, Hao Liu, Zhe-wei Zhang, Ji-min Chen
European Journal of Medical Research.2023;[Epub] CrossRef - Classification for Staging and Managing Patients with Biopolymer-induced Human Adjuvant Disease
Jaime Eduardo Pachón Suárez, Marcela C. Salazar, Victor Z. Rizo
Plastic and Reconstructive Surgery - Global Open.2022; 10(2): e4137. CrossRef - Development of Metabolic Synthetic Lethality and Its Implications for Thyroid Cancer
Sang-Hyeon Ju, Seong Eun Lee, Yea Eun Kang, Minho Shong
Endocrinology and Metabolism.2022; 37(1): 53. CrossRef - Drug delivery for metabolism targeted cancer immunotherapy
Taravat Khodaei, Sahil Inamdar, Abhirami P. Suresh, Abhinav P. Acharya
Advanced Drug Delivery Reviews.2022; 184: 114242. CrossRef - Sulfur quantum dot based fluorescence assay for lactate dehydrogenase activity detection
Shengnan Fan, Xiaoqing Li, Fanghui Ma, Minghui Yang, Juan Su, Xiang Chen
Journal of Photochemistry and Photobiology A: Chemistry.2022; 430: 113989. CrossRef - STAT3/LINC00671 axis regulates papillary thyroid tumor growth and metastasis via LDHA-mediated glycolysis
Nan Huo, Rui Cong, Zhi-jia Sun, Wen-chao Li, Xiang Zhu, Chun-yuan Xue, Zhao Chen, Lu-yuan Ma, Zhong Chu, Yu-chen Han, Xiao-feng Kang, Song-hao Jia, Nan Du, Lei Kang, Xiao-jie Xu
Cell Death & Disease.2021;[Epub] CrossRef
|